Many students find the task of memorizing molecular shapes daunting, especially when faced with numerous combinations and possibilities😵💫
I've heard this concern over the years: “How do I remember all these shapes and their variations?” It's a common challenge, but fear not – the key lies in understanding the VSEPR theory!
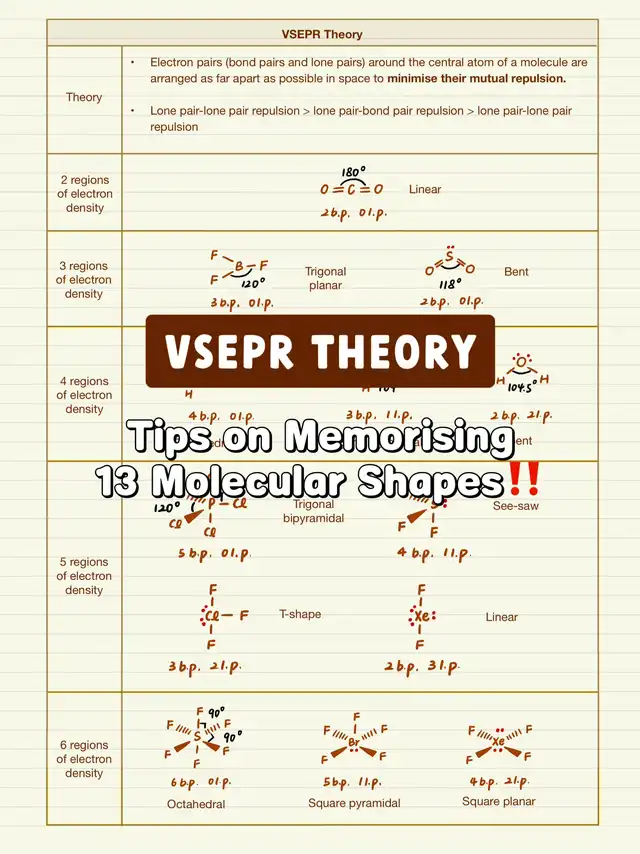
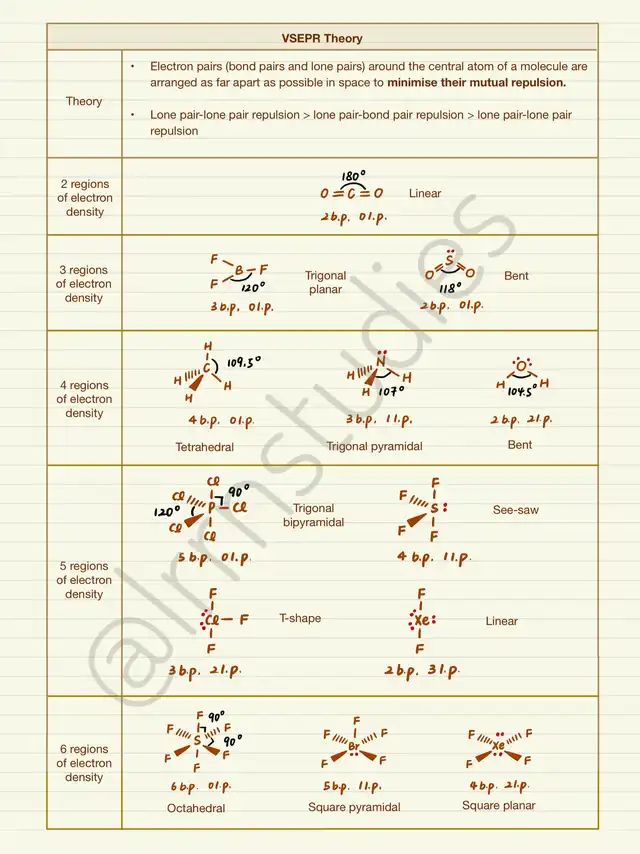
⭐️ The core idea is that the arrangement of bond pairs is influenced by repulsion, with LOWER repulsion leading to GREATER stability. Since lone pair-lone pair repulsion is the greatest, the priority is to minimize such repulsion, followed by lone pair-bond pair repulsion, and lastly bond pair-bond pair repulsion.
⭐️ Electron pairs (bond pairs + lone pairs) can be categorized into five series (‼️multiple bond is considered as ONE bond pair):
👉 Two pairs: Linear with a bond angle of 180 degrees.
👉 Three pairs: Trigonal planar with a bond angle of 120 degrees. When there is one lone pair, the bond pairs form a V-shape/bent shape due to the mutual repulsion of bond pairs and lone pair, resulting in a decreased bond angle.
👉 Four pairs: Tetrahedral with a bond angle of 109.5 degrees. For each additional lone pair, the bond angle decreases by 2.5 degrees. To remember the shape, try visualizing the 3D structure outlined by the bond pairs. For instance, with one lone pair and three bond pairs, the three bond pairs form a pyramid with a trigonal base. Hence, the shape is called trigonal pyramidal.
👉 Five pairs: Trigonal bipyramidal with a trigonal planar center (equatorial plane) and bond angles of 120 degrees within the equatorial plane. Due to the relatively large bond angle in the equatorial plane, lone pairs are placed here to minimize repulsion, forming a seesaw shape when viewed horizontally, and T-shape and linear shapes.
👉 Six pairs: Octahedral with a square planar center and bond angles of 90 degrees within the plane. When there is one lone pair, placing it in the plane or above/below the plane makes no difference, resulting in a square pyramidal shape. With two lone pairs, minimizing lone pair-lone pair repulsion takes priority, placing them above and below the plane to reduce repulsion, forming a square planar shape.
⭐️ Technically, remembering the first shape in each series is sufficient. The arrangements of lone pairs can be deduced based on the principle of reducing repulsion, and the arrangement of bond pairs gives the molecular shape.
⭐️Remember, it's not about memorization; it's about comprehension.
#jc #studymotivation #StudyTips #studying #studentfriendly #studentlife #studyessentials #studywithme #studyhacks #study